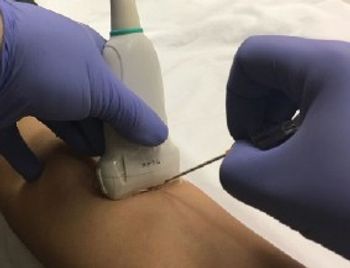
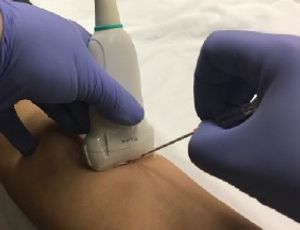
Accurate device information and proper sterilization or disinfection are crucial to ensure safety during ultrasound procedures.

Lisa Waldowski, DNP, RN, CIC, is an expert in infection prevention and control with more than 30 years of experience in the health care industry. She recently joined Wellstar Health System in Atlanta, Georgia, as system executive director of infection prevention and control. Waldowski holds a doctor of nursing practice in systems leadership and a master of science in nursing. She is board certified in infection prevention and control.

Accurate device information and proper sterilization or disinfection are crucial to ensure safety during ultrasound procedures.

Read about the sterilization process of eye instruments and devices.

Eye devices often are not cleaned nor sterilized as they should be according to manufacturer’s instructions for use, which leads to poorer patient outcomes.
The high-level disinfection process of an ultrasound probe, when indicated, includes documentation that demonstrates high-level disinfection was performed, and patient identifiers were documented to link the ultrasound probe to the patient. This is often referred to as traceability.
Published: July 23rd 2021 | Updated:
Published: May 19th 2023 | Updated:

Published: December 28th 2022 | Updated: